#API meaning pharma
Explore tagged Tumblr posts
Text
Your Ultimate Partner in Medicine Manufacturing and Supply Chain Excellence
Chemxpert Database empowers pharmaceutical professionals by offering extensive insights into medicine manufacturing, including connections with top China pharmaceutical companies. With a focus on sterile manufacturing and rigorous pharmaceutical testing, Chemxpert streamlines supply chain management in the pharmaceutical industry, ensuring quality and compliance at every stage. Trusted for its robust data, Chemxpert Database is an invaluable tool for enhancing manufacturing efficiency and maintaining high standards across global pharmaceutical operations.
#pharmaceutical compliance#low cost medicine#pharmacy distributor#API meaning pharma#top pharma companies in USA#contract manufacturing pharma#pharma development
1 note
·
View note
Text
Here’s how companies can ensure they’re in compliance with new requirements that go into effect in August. Nitrosamine contamination has become one of the most pressing issues facing the pharmaceutical industry today. In the past few years, these potentially cancer-causing substances have grabbed the public’s attention and become a major focus of regulators, prompting the adoption of rigorous new FDA guidelines that will go into effect this summer. Since 2018, the FDA has issued more than 500 recalls because of unacceptable levels of nitrosamines in active pharmaceutical ingredients (APIs). As executive director of analytical at SK Pharmteco, I work with pharmaceutical clients to provide comprehensive testing services for a broad range of compounds, including impurities such as nitrosamines. Based on what we’re seeing, it’s clear that the spotlight on nitrosamines will only get brighter with manufacturers having to meet new FDA guidelines for these compounds by August. The new FDA guidelines, issued last year, are both comprehensive and complex. They cover more than 250 nitrosamine compounds and apply to every drug on the market or in development. These guidelines require detecting minute levels—down to the parts per billion—with limits that vary depending on the specific nitrosamine. The stakes are high for getting it right. Noncompliance triggers an FDA Class II recall, which means drugs have to be immediately pulled from shelves. The FDA will not approve new drugs unless they meet the guidelines. Compliance may necessitate reformulation of products with different excipients and inputs. Additionally, it could require redesigning production processes, as nitrosamines can form through chemical reactions between precursors that are otherwise not hazardous on their own. In light of these stakes, pharma executives must ensure they have a robust testing program in place now and are proactively addressing any issues with noncompliant nitrosamine levels. A Fast-Developing Issue Nitrosamines can be found in a wide range of products, from foods and beverages to cosmetics and pharmaceuticals. These compounds are created when amines react with nitrosating agents such as nitrous acid, nitrogen oxides and nitrites. Nitrosamines were observed by chemists in the late 1800s, but the dangers were not understood at the time. In the 1970s, William Lijinsky, laboratory director at the National Cancer Institute’s Frederick Cancer Research and Development Facility, became a leading voice in raising concerns about their link to cancer. Initial concerns focused on tobacco products and foods, such as processed meats, where smoking or curing can introduce nitrites to trigger the chemical reaction. In the following decades, concerns grew as more studies indicated a link between different nitrosamines and cancer. Studies also showed that even small amounts of nitrosamines could be dangerous, leading regulators and experts to place greater scrutiny on their presence in products such as pharmaceuticals. In 2018, the FDA began an investigation into nitrosamine impurities in drugs that led to high-profile recalls for heartburn medicines. In 2020, the FDA issued its first guidance on the compounds, making nitrosamines an issue for the entire pharmaceutical industry for the first time. The FDA’s actions corresponded with increasing public concern about potential carcinogens in everything from food to toys and even water. While deaths from cancer have declined in the U.S., the number of new cases continues to climb each year, according to the American Cancer Society. The existence of nitrosamines in prescription drugs is particularly troubling because the medications are intended to treat illnesses, not cause them. They’re also intended to be taken on a regular basis. The FDA’s rapid and comprehensive response to the nitrosamine issue signals that it has taken a place among the agency’s top priorities in protecting the public. SK Pharmteco has seen pharma’s quick response to this change. ...
0 notes
Text
Turnkey Pharmaceutical Projects: The Smart Approach to Efficient and Compliant Pharma Setup

Setting up or expanding a pharmaceutical manufacturing facility is a complex process. It requires precision, compliance, expertise, and speed to meet the ever-increasing demand for quality medicines. This is where turnkey pharmaceutical projects offer a game-changing solution. These end-to-end services provide everything a pharmaceutical company needs—from facility design and equipment installation to commissioning and validation—under a single roof.
By choosing pharma turnkey projects, companies eliminate the risks of dealing with multiple vendors, miscommunication, and project delays. Instead, they benefit from a streamlined, coordinated, and compliant setup that meets regulatory standards and supports long-term scalability.
What Are Turnkey Pharmaceutical Projects?
Turnkey pharmaceutical projects are comprehensive, ready-to-use solutions that include the full scope of planning, execution, and delivery of pharmaceutical manufacturing facilities. The term "turnkey" means the client simply needs to "turn the key" and start operations once the provider completes the setup.
The scope of pharma turnkey projects typically includes:
Feasibility studies and site analysis
Conceptual and detailed engineering design
Civil construction and infrastructure development
HVAC and cleanroom installation
Procurement and installation of pharmaceutical-grade equipment
Automation and control systems integration
Validation, qualification, and regulatory documentation
Training and project handover
With this approach, pharmaceutical companies can focus on production, quality assurance, and business strategy, leaving the technical complexities to experienced turnkey providers.
Benefits of Turnkey Pharmaceutical Projects
1. Single Point of Responsibility
Working with a single vendor for the entire project ensures clear accountability. This minimizes coordination errors and provides the client with peace of mind throughout the execution of the pharma turnkey project.
2. Time and Cost Efficiency
By integrating all phases of the project under one provider, timelines are reduced, and cost control becomes more effective. There are fewer chances of miscommunication, procurement delays, or design changes that can inflate budgets.
3. Regulatory Compliance
A reliable turnkey provider understands global and local pharmaceutical regulations, such as WHO-GMP, USFDA, EU-GMP, and PIC/S. They ensure that every element of the facility meets required compliance standards, reducing the risk of non-conformity or costly rework.
4. Custom-Built Solutions
Every pharmaceutical company has unique requirements based on the products they manufacture. Whether it's oral solid dosage, sterile injectables, or APIs, turnkey pharmaceutical projects are tailored to meet the specific needs of the client’s manufacturing processes.
5. Faster Time to Market
With optimized project timelines and integrated workflows, companies can get their facilities up and running faster. This is especially critical in competitive markets or during healthcare emergencies when rapid production scale-up is essential.
Key Components of a Pharma Turnkey Project
To understand the true value of pharma turnkey projects, it’s essential to break down their core components:
a) Cleanroom Design and HVAC Systems
Contamination control is central to pharmaceutical manufacturing. Cleanroom layout, airflow design, and HVAC systems are engineered to maintain specific environmental conditions. Ergonomic and compliant cleanroom designs enhance productivity and reduce operational risks.
b) Pharmaceutical Equipment Installation
Turnkey providers source, install, and calibrate a wide range of pharmaceutical equipment, from mixers and granulators to tablet presses and filling lines. All installations are performed according to cGMP guidelines.
c) Automation and Data Integration
Modern turnkey pharmaceutical projects integrate automation and smart systems to ensure precise process control, real-time monitoring, and better data traceability—essential for quality assurance and regulatory audits.
d) Documentation and Validation
The provider ensures that all critical systems and processes are validated, and the necessary documentation is in place. This includes IQ, OQ, PQ protocols and Standard Operating Procedures (SOPs), helping clients achieve regulatory approval swiftly.
Why Pharma Companies Prefer Turnkey Projects
Pharmaceutical companies today operate in a highly competitive and regulated environment. Delays in facility setup can mean missed opportunities and increased costs. With pharma turnkey projects, companies benefit from a hassle-free experience, faster execution, and long-term operational excellence.
Moreover, as markets expand globally, turnkey solutions support scalability and modernization. Whether it’s setting up a new production unit or upgrading an existing one, turnkey pharmaceutical projects are the most effective way to ensure quality, compliance, and speed.
Choosing the Right Turnkey Partner
Not all turnkey providers are equal. When selecting a partner for pharma turnkey projects, companies should evaluate:
Industry experience and technical expertise
Compliance knowledge and past project success
Ability to customize solutions
After-sales support and training
Project timeline and budget management
A good turnkey partner acts not just as a vendor but as a strategic consultant who helps clients achieve their long-term production goals.
Conclusion
Turnkey pharmaceutical projects are revolutionizing how pharma companies build, expand, and modernize their production capabilities. By offering complete, compliant, and ready-to-operate facilities, these projects minimize risk, reduce delays, and optimize resources. For pharmaceutical businesses aiming to stay competitive, efficient, and compliant, investing in pharma turnkey projects is not just a smart choice—it’s a strategic necessity.
#pharma turnkey projects#Turnkey pharmaceutical projects#pharma consultancy#cleanroom construction#biotech management consulting#pharmaceutical turnkey projects#pharmaceutical consultant#biopharmaceutical consulting#turnkey solutions#cleanroom design#pharmaceutical consultancy services
0 notes
Text
How a Riyadh-Based Company is Revolutionizing Logistics Tracking
In today’s fast-paced world, logistics is no longer a support function — it’s a growth driver. Across the globe, businesses are investing heavily in smarter ways to manage deliveries, monitor fleets, and improve their customer experience. But in the heart of Riyadh, one company is setting new standards in how logistics tracking is being done.

Meet Five Programmers, a local software powerhouse that's changing the way businesses in Saudi Arabia approach their logistics operations. With custom-built solutions tailored to the region's unique needs, they’re redefining what’s possible in the supply chain sector.
The Digital Shift in Riyadh’s Logistics Sector
As the capital of Saudi Arabia, Riyadh is not just a political hub — it’s a growing logistics hotspot. Thanks to Vision 2030 and the rise of e-commerce, demand for real-time, tech-driven solutions is accelerating.
Businesses here face challenges like:
Urban traffic congestion
Delivery delays due to route inefficiencies
Limited visibility into vehicle performance
Lack of centralized data systems
To overcome these hurdles, companies are seeking smarter tools that offer more than just basic GPS tracking.
Local Challenges Need Local Solutions
Most international logistics platforms are designed with global use cases in mind. They rarely address region-specific pain points, such as Arabic-language support, local compliance laws, or road network behaviors within Riyadh.
This is where a homegrown player like Five Programmers steps in — with a deep understanding of both the technical and local business environments.
Their solutions don’t just track movement. They provide intelligence.
What Makes Five Programmers Different?
Rather than offering a one-size-fits-all software, Five Programmers focuses on building customized logistics tracking platforms that adapt to your exact operational needs.
Some of the standout features of their systems include:
🔹 Real-Time Vehicle Visibility
Track all delivery vehicles on a single interactive map. See where your assets are, estimated arrival times, and current status in real time.
🔹 Route Optimization Algorithms
Their solution identifies the most efficient paths using local traffic data — reducing fuel consumption and delivery time.
🔹 Smart Alerts & Notifications
Get notified when a vehicle deviates from its route, is idle too long, or enters/exits a geo-fenced area.
🔹 Multilingual Interfaces
Designed for local teams, their dashboards support both Arabic and English, making adoption easy across your workforce.
🔹 Integration-Ready
Need to connect with your existing ERP, CRM, or warehouse systems? No problem. They provide seamless integrations via APIs.
Industries That Benefit in Riyadh

The demand for better logistics tracking is spreading across various sectors in Riyadh, including:
E-Commerce: Improve delivery reliability and customer satisfaction
Pharma & Medical Supply: Ensure temperature-sensitive goods are safely delivered
Construction: Schedule materials and equipment with precision
Retail Chains: Manage store replenishment efficiently
In each of these verticals, better tracking leads to better performance.
Aligning with Vision 2030 Goals
Saudi Arabia’s Vision 2030 emphasizes digital innovation, operational efficiency, and reduced dependency on manual processes. For logistics firms in Riyadh, this means one thing: adapt or fall behind.
By developing modern tracking systems locally, Five Programmers is helping companies embrace change without friction. Their tools are built with compliance in mind and can easily scale as the business grows.
Case Study: A Riyadh-Based Courier Company
A medium-sized courier company in Riyadh partnered with Five Programmers to digitize their entire delivery process. Before that, they relied on phone calls and spreadsheets to monitor deliveries.
After implementing the new platform:
On-time delivery rates improved by 34%
Customer complaints dropped by 41%
Fleet fuel usage decreased by 22%
Managers had live insight into driver performance
These results aren’t just numbers — they reflect real business transformation made possible by smarter tools.
Technology That Powers the Change
Behind the scenes, Five Programmers uses cutting-edge technologies like:
Cloud hosting for 24/7 access
AI-based route planning
Custom analytics dashboards
Secure data encryption
Yet the real power lies in their ability to customize. Whether you run five vehicles or five hundred, the platform is designed to grow with your needs.
Focus on Support and Service
One of the main reasons clients in Riyadh prefer Five Programmers is their commitment to ongoing support. Unlike offshore providers, this local team is available to:
Offer on-site or remote training
Provide real-time issue resolution
Roll out updates based on Saudi market trends
This personalized approach makes it easier for companies to adopt and benefit from the software quickly.
Looking Ahead: The Future of Logistics in Riyadh

The logistics space in Saudi Arabia is evolving fast. Concepts like drone deliveries, warehouse automation, and AI-based inventory systems are no longer futuristic ideas — they’re on the horizon.
With tools from Five Programmers, businesses in Riyadh are laying the groundwork to integrate with such innovations in the near future.
By investing in logistics tracking today, companies are not just solving current problems — they’re preparing for a smarter, faster, and more agile future.
Final Words: Innovation That Moves with You
In the competitive world of logistics, visibility is everything. Companies that know exactly where their goods are — and how to optimize movement — are the ones that stay ahead.
With a powerful partner like Five Programmers, Riyadh-based businesses don’t have to rely on foreign platforms that lack local relevance. They can now build smarter tracking solutions, tailored to their workflows, their people, and their goals.
In a city that never stops moving, isn’t it time your logistics moved smarter?
Quick FAQ
Q: Is this logistics tracking solution mobile-friendly? Yes, the platform is responsive and works across smartphones, tablets, and desktops.
Q: Can it work for delivery teams across different cities? Absolutely. While the company is based in Riyadh, the platform supports multi-city operations.
Q: Do I need to replace my current systems? Not necessarily. Five Programmers offers integration options so you can keep your existing ERP or CRM.
Q: How long does implementation take? Most mid-sized clients go live within 2–3 weeks, depending on customization needs.
#mobile app development company in saudi arabia#logistics management software#flutter app development company in riyadh#android app development#100 days of productivity#ai driven logistics platform in riyadh#logistics mobile app development in riyadh#logistics app development
0 notes
Text
Raising the Bar in Industrial Safety and Compliance: The Veera Group Way
In high-stakes industrial environments, safety, quality, and compliance aren't just goals—they’re essentials. Industries today face growing regulatory scrutiny, workforce safety concerns, and client expectations for flawless execution. At Veera Group, safety and quality assurance are not afterthoughts—they’re embedded into every project, every process, and every piece of equipment.
With its roots in precision engineering and a commitment to global standards, Veera Group ensures that all systems, from process plants to modular skids, are built to operate safely, efficiently, and reliably under the most demanding conditions.
Safety First, Always
Industrial operations often involve hazardous materials, high pressures, extreme temperatures, or flammable substances. Veera Group takes a proactive approach to safety, incorporating hazard assessments and fail-safe mechanisms from the design stage itself. Every project undergoes a rigorous HAZOP (Hazard and Operability) study, ensuring potential risks are identified and mitigated early on.
From explosion-proof control panels to automated emergency shutdown systems, Veera Group integrates technology and engineering controls that prioritize worker and process safety.
To learn more about the company’s approach to safe, reliable engineering, visit the Veera Group homepage.
Built to Comply with the Toughest Standards
Compliance is more than meeting legal checkboxes—it’s about building systems that stand up to global scrutiny. Veera Group fabricates its systems to align with international codes and standards such as:
ASME (Boiler & Pressure Vessel Code)
API (American Petroleum Institute)
ATEX (for explosive atmospheres)
CE Marking (for European market compliance)
GMP (Good Manufacturing Practices) for pharma and food industries
All fabrication is backed by documented quality assurance procedures, third-party inspections, and client validation protocols. Veera Group’s in-house team ensures that every weld, control loop, and instrumentation system meets or exceeds compliance requirements—before the system ever leaves their facility.
Digital Monitoring for Better Oversight
Modern compliance also means traceability and documentation, and Veera Group leverages digital tools to deliver just that. From digital weld logs to sensor-based performance monitoring, their systems are designed to give plant operators and auditors real-time access to key safety and quality data.
In automated facilities, operators can track temperature, pressure, flow rates, and other critical variables from a central HMI or SCADA interface—helping detect anomalies early and avoid costly failures.
Sustainability Through Safe Handling
Sustainability is closely tied to safe operations, particularly when it comes to waste management. One area where Veera Group is making a significant impact is in used motor oil recycling. Improper handling of used oil can result in severe environmental hazards and penalties.
Veera Group’s recycling units are designed with multiple safety features: leak-proof construction, temperature and pressure controls, vapor recovery systems, and fire suppression options. These help clients meet strict environmental regulations while turning waste into value.
To understand how these safe and eco-friendly systems work, explore their detailed Used Motor Oil Recycling: 2025 Guide.
Sharing Knowledge to Build Safer Futures
Veera Group doesn’t just implement safety—they also spread awareness. Their blogs section regularly covers safety innovations, compliance tips, and updates on industrial best practices. Whether you're looking to prepare for a regulatory audit or train your team on risk mitigation, the blog is a go-to resource.
Why Clients Trust Veera Group
Across industries and continents, clients choose Veera Group because they deliver more than equipment—they deliver confidence. With zero-compromise policies on safety and quality, and a dedicated team of experts ensuring every detail is handled, Veera Group remains a trusted partner for mission-critical projects.
Conclusion: In industrial environments where every detail matters, Veera Group leads with safety, compliance, and quality. Their proactive approach ensures your plant isn’t just productive—it’s protected. When you’re building for the future, build it safely—with Veera Group.
0 notes
Text
Jay Finechem: A Leading Global Supplier of API Intermediates
In today’s highly regulated pharmaceutical landscape, finding a reliable global supplier of API intermediates is more than a procurement decision—it’s a strategic partnership. With the increasing complexity of drug development and rising demand for quality pharmaceutical ingredients, companies around the world are turning to trusted names like Jay Finechem. Renowned for its technical expertise, global reach, and regulatory excellence, Jay Finechem has earned its place among the top API intermediate exporters from India.
Trusted API Intermediate Solutions for the Pharma Industry
Jay Finechem is a specialized GMP certified API manufacturer offering a broad portfolio of high-purity pharma intermediates to pharmaceutical companies worldwide. Whether you're sourcing intermediates for cardiovascular drugs, oncology products, or antibiotics, our team ensures every batch meets stringent international standards. As a global API manufacturer, we maintain consistency, traceability, and compliance throughout our production processes.
Custom API Manufacturing and End-to-End Services
What sets Jay Finechem apart from other API intermediates manufacturers is our flexibility in offering custom API manufacturing services. With dedicated R&D and pilot-scale capabilities, we support custom synthesis for APIs as per clients’ specifications. Our integrated approach—from development to delivery—allows us to offer end-to-end API production services that are cost-effective and time-efficient.
From small-scale innovation projects to full-scale commercial production, Jay Finechem is equipped to handle it all. This has made us a preferred partner for pharmaceutical intermediates suppliers and bulk drug intermediates manufacturers across the globe.
Why Global Clients Choose Jay Finechem
Being a global supplier of API intermediates means more than just exporting products—it means delivering reliability, regulatory support, and on-time delivery to partners in regulated markets like the US, EU, and Southeast Asia. Jay Finechem offers:
Regulatory compliant chemical supply
Comprehensive documentation support for audits and filings
Robust logistics for global distribution
Consistent quality assurance at every stage
Our reputation as a dependable API contract manufacturer is built on long-term collaborations and a clear understanding of each market’s requirements.
India's Trusted Partner in Global Pharma Supply Chains
Operating from India, Jay Finechem has become a prominent Indian API intermediates supplier known for its world-class infrastructure and cost competitiveness. Our positioning among API intermediate companies is strengthened by our ability to deliver pharma-grade intermediates at scale, making us a go-to solution for intermediates for drug manufacturing.
Partner with a Proven Leader
In an industry where precision, compliance, and trust matter, Jay Finechem stands tall as a global supplier of API intermediates you can rely on. Our global footprint, innovative mindset, and commitment to quality make us a true partner in pharmaceutical success.
Ready to streamline your supply chain with a trusted name in API intermediates? Partner with Jay Finechem—where quality meets reliability, globally.

0 notes
Text
Pharmaceutical Intermediates: The Backbone of Drug Manufacturing | Chemox Pharma
Pharmaceutical Intermediates play a key role in the drug manufacturing process. At Chemox Pharma we are specialists in the production of high quality pharmaceutical intermediates which we see as the basic elements that go into Active Pharmaceutical Ingredients (API’s). These materials are vital in the creation of the end medicinal product which is delivered to patients world wide.

What Are Pharmaceutical Intermediates?
Pharmaceutical intermediates are what we see in between the start and the end products in the production of Active Pharmaceutical Ingredients. These are not active by themselves but serve as the base for a series of chemical transformations which in the end produce the pure drug product. At times these intermediates are isolated out of the process and at other times they are left as they are which is fine in a multi step synthesis of large scale drug production.
They are integral to the pharma supply chain which we at Chemox Pharma have designed for precision, consistency and safety of our drugs. Our intermediates at Chemox Pharma conform to the highest global quality standards and we manufacture in world class facilities that are within the strictest regulatory parameters.
API vs Intermediate: Comprehending the Difference
In the pharma industry a frequent question is that of what an API and an intermediate are. Although both are key to drug production they have different roles.
API (Active Pharmaceutical Ingredient): This which we put in a drug that which has health benefits is the active element that addresses a health issue.
Pharmaceutical Intermediate: This is a starting material which we use in the production of the API. It is formed during the synthesis process and goes through more chemical changes before becoming the final API.
In other words, intermediates are the stages which a drug goes through to become a final product and APIs are what we get at the end.
Role of Intermediates in Pharma
In the pharmaceutical industry intermediates serve as the base for efficient, cost effective and scalable drug production. They enable the development of complex molecules in a piecewise and controlled way. Also this which in turn reduces production time and at the same time improves the purity and quality of the final drug.
Also it is of great importance that pharma companies source high quality intermediates. At Chemox Pharma we see this as a key issue which is why we present to you a reliable supply of certified pharmaceutical intermediates which we have tailored to cover the wide range of what our global markets require.
Bulk Drug Intermediates: Rising demand for.
With growing demand for generic and branded drugs the need for bulk drug intermediates is also on the rise. We see large scale production of these intermediates which also tend to be customized to fit the unique synthesis needs of different Active Pharmaceutical Ingredients.
Chemox Pharma is at the leader in this field, we supply bulk pharmaceutical intermediates for a wide range of therapeutic areas which include cardiovascular, anti infective, anti inflammatory, and CNS drugs. We are committed to innovation and quality which in turn means our partners’ access to intermediates that meet international standards and support smooth API development.
Intermediate Chemicals: Quality we stand behind.
The production of intermediate chemicals we see to be a very technical process which requires in depth knowledge, large scale infrastructure, and a great deal of experience with chemical synthesis. At Chemox Pharma we have that experience and we are able to present to you superior quality intermediates that are also safe, consistent and efficient.
Our R&D team is at the go go pace to improve synthesis routes, reduce impurities, and see to it that we are in regulatory compliance. We in turn see to it that our clients’ production processes run more smoothly which in term allows them to bring high quality drugs to market faster.
Why Choose Chemox Pharma?
Regulatory Compliance: Our manufacturing processes adhere to cGMP standards and international regulatory bodies’ rules.
Global Supply Chain: Global supply and timely delivery of pharmaceutical intermediates.
Expertise & Innovation: Supported by a team of expert scientists and chemical engineers.
Conclusion
Pharmaceutical intermediates are the backbone of modern medicine. In API synthesis and mass drug production they play a key role which cannot be ignored. At Chemox Pharma we are focused on bringing to you superior quality pharmaceutical intermediates which also include bulk drug intermediates and intermediate chemicals we do with excellence in reliability and precision.
For our mid size needs we turn to Chemox Pharma your trusted name in API and intermediate manufacturing.
0 notes
Text
Quinine Base: Essential API for Modern Antimalarial Therapies
Introduction: The Critical Function of Quinine Base in Treating Malaria
For centuries, malaria has been a leading cause of death in the world. But with the advancement of pharmaceutical science, the treatments have become more effective and affordable. Among the various essential solutions is quinine base, an age-old compound that still remains at the forefront of antimalarial treatments. Prism Industries Pvt. Ltd. specializes in the production of high-quality quinine base and is the sought-after quinine base manufacturer catering to healthcare across the globe.

As a leading manufacturer of active pharmaceutical ingredients, we take pride in facilitating global malaria management efforts through the supply of quality API Bulk Drugs such as quinine base for use in formulations.
Knowing Quinine Base: A Historical and Contemporary Insight
Quinine base is an alkaloid extracted from the Cinchona bark. Traditionally employed by South American indigenous peoples, Europeans discovered quinine's medicinal value in the 17th century. Speed forward to the present, and quinine base still forms part of the main ingredients used in combating malaria, particularly drug-resistant types.
With the strong quinine alkaloid structure, quinine base acts against the parasite in the manner described by inhibiting the parasite to break down the hemoglobin for digestion, killing it in the process and enabling malaria patients' recovery.
Extracting and processing quinine base at Prism Industries Pvt. Ltd., we undertake state-of-art processing that helps in yielding an extremely pure, stable active pharma ingredient matching the rigorous specs of world pharmacy firms.
Solubility: Slightly soluble in water, soluble in alcohol.
Stability: Very stable when stored under standard conditions.
Uses: Mainly applied to quinine for treating malaria, but also studied for other therapeutic uses.
Our high-purity quinine base provides consistent quality, which guarantees its optimal performance in various pharmaceutical uses.
How Quinine Base Functions in Malaria Therapy
In malaria treatment, quinine base attacks the blood stages of Plasmodium parasites, particularly Plasmodium falciparum, which causes the most deadly forms of the disease. Quinine base affects the parasite's mechanism of metabolizing and detoxifying heme, causing it to accumulate and ultimately kill the parasite.
Since its life-sustaining mechanism, quinine base continues to be a necessity, especially where newer antimalarial drugs meet resistance problems.
At Prism Industries Pvt. Ltd., we take care to thoroughly test the quinine base we manufacture to ensure therapeutic efficacy for quinine used in the treatment of malaria.
Manufacturing Excellence at Prism Industries Pvt. Ltd.
Being a leading quinine base manufacturer and among the top active pharmaceutical ingredient manufacturers in India, Prism Industries Pvt. Ltd. follows the finest manufacturing practices. Our in-house manufacturing facility is supported by:
WHO-GMP Certification
Compliance with ISO Standards
Sophisticated R&D Labs
Stringent Quality Assurance Programs
Our dedication to quality means that our API Bulk Drugs, such as quinine base, are contaminant-free, impurity-free, and meet pharmacopeial standards.
Applications Outside Malaria: The Growing Potential of Quinine Base
While quinine for the treatment of malaria is still the main application, scientists are investigating new areas of therapy where quinine base has potential, such as:
Treatment of nocturnal leg cramps.
Potential antiviral uses.
Investigation into autoimmune disease management.
With increasing demand comes increasing demand for a reliable quinine base producer such as Prism Industries Pvt. Ltd., committed to supplying top-grade quinine alkaloid solutions for new pharmaceutical requirements.
API Bulk Drugs and the Role of Reliable Supply Lines
For the pharmaceutical sector, availability of top-grade API Bulk Drugs such as quinine base is an imperative. Delays or irregularities can slow down drug manufacture and undermine treatment accessibility.
At Prism Industries Pvt. Ltd., we understand the significance of:
Consistency: Consistent quality in each batch.
Scalability: Capacity to handle large volumes without sacrificing standards.
Timely Delivery: Effective logistics to cater to global markets.
Regulatory Compliance: Complete documentation for regulatory filings.
This dependability makes us a valued partner among leading active pharmaceutical ingredient manufacturers globally.
Why Choose Prism Industries Pvt. Ltd. as Your Quinine Base Manufacturer?
In the matter of procuring quinine base, collaboration with a skilled, quality-oriented producer is paramount. Prism Industries Pvt. Ltd. is remarkable in many ways:
Decades of Experience: Depth of experience in manufacturing quinine alkaloid APIs.
World-Class Facilities: Advanced infrastructure for clean and efficient production.
Customer-Focused Approach: Tailor-made solutions designed according to your exact requirements.
Competitive Pricing: Value without compromise on quality.
Our dedication to excellence has earned us a reputable name among Indian and global active pharmaceutical ingredient manufacturers.
Global Market Demand and Trends for Quinine Base
Demand for quinine base remains high, especially in:
Sub-Saharan Africa
Southeast Asia
South America regions
These regions face the largest malaria burdens and hence quinine for treatment of malaria is an important pharmaceutical requirement. In addition, greater research on the use of quinine alkaloid is creating new market possibilities.
Being the major suppliers of API Bulk Drugs, we at Prism Industries Pvt. Ltd. are expanding our operations to address these growing needs in a sustainable and responsible manner.
Our Commitment to Sustainability and Innovation
Sustainability is not a choice anymore; it's a duty. At Prism Industries Pvt. Ltd., we take care to:
Eco-Friendly Practices: Minimization of waste and eco-friendly manufacturing processes.
Ethical Sourcing: Ethical procurement of natural resources.
Continuous Innovation: R&D investment for enhancing manufacturing effectiveness and creating future-ready APIs.
Our mission is to be a pioneer among active pharmaceutical ingredient manufacturers by blending tradition, innovation, and sustainability.
#quinine_base#quinine alkaloid#custom_api_manufacturing#api_pharma_company#prism_industries_Pvt._Ltd.
0 notes
Text
The Microbiome of Outer Space: How Space Travel Changes Human Gut Bacteria

When astronauts leave Earth, they don’t just take their training and equipment with them—they also carry trillions of microbes inside their bodies. The gut microbiome, essential for digestion, immunity, and overall health, experiences notable changes during space travel. Scientists, including experts in R&D in pharmaceuticals, are now uncovering how microgravity, radiation, and an altered diet impact the balance of gut bacteria and what that means for long-term missions.
A Journey Beyond Earth: What Happens to Gut Bacteria in Space?
Space presents an entirely new environment for the human body, and its effects on the microbiome are profound. Scientists, including pharmaceutical solutions providers, have discovered essential factors influencing changes in gut bacteria:
Microgravity Disrupts Gut Function – How fluids and nutrients move through the digestive system changes in a low-gravity environment, potentially altering the gut’s bacterial ecosystem.
Radiation Alters Microbial Diversity – Exposure to cosmic radiation damages cells, including those lining the intestines, which can impact microbial populations. Biopharmaceuticals suppliers are researching protective measures to minimize these effects.
Space Food Lacks Variety – Astronauts eat a carefully prepared but fiber-deficient diet, which may reduce beneficial bacteria that thrive on plant-based nutrients. Pharma quality assurance plays a role in ensuring that food supplements meet the highest safety standards for space travel.
A Sterile Spacecraft Environment – Unlike on Earth, astronauts have limited exposure to diverse microbes from soil, air, and fresh foods, potentially leading to an imbalance in their gut flora.
The Health Consequences of an Altered Microbiome
Scientists are only beginning to understand the full impact of these microbiome changes. However, some potential consequences include:
Immune System Weakness – A disrupted microbiome may reduce the body’s ability to fight infections, leaving astronauts more vulnerable to illnesses.
Digestive Disruptions – An imbalance in gut bacteria can lead to digestive discomfort, irregular bowel movements, and nutrient absorption issues.
Mental Health Implications – The gut-brain connection suggests that microbiome imbalances could influence mood, stress levels, and cognitive function. Pharmaceutical innovation partners are exploring ways to develop microbiome-based treatments to mitigate these effects.
Inflammation and Long-Term Health Risks – Changes in gut bacteria could contribute to increased inflammation, which has been linked to chronic diseases. API quality control ensures that medications developed to counteract these risks maintain safety and effectiveness.
Can We Protect the Astronaut Microbiome?
To mitigate these risks, researchers, including experts at Rang Life Sciences, are exploring potential solutions:
Probiotic and Prebiotic Supplements – Introducing beneficial bacteria or fiber-rich supplements may help maintain a balanced gut environment.
Improved Space Diets – Scientists are developing nutrient-rich, microbiome-friendly meals to support astronaut health on extended missions.
Real-Time Microbiome Monitoring – Regular gut bacteria testing during space travel could allow for early intervention when imbalances are detected.
A Step Toward the Future
Studying the microbiome in space isn’t just about keeping astronauts healthy—it also provides insights into gut health here on Earth. Understanding how extreme environments affect microbial ecosystems could lead to advancements in treating digestive disorders, immune diseases, and mental health conditions.
As humanity pushes further into space, taking care of the microbiome will be as important as managing oxygen, water, and food supplies. Thanks to ongoing research by pharmaceutical solutions providers, the future of space travel may very well depend on the tiny, invisible allies within us.
0 notes
Text
Barcode Definition 2025: How They Work in Modern Times

Barcodes have been a part of global commerce and logistics for decades—but in 2025, they’ve evolved far beyond basic black-and-white stripes on a product package. Today’s barcodes are smarter, faster, and more integrated into digital systems than ever before, playing a key role in supply chain automation, inventory management, retail checkouts, healthcare, and even customer engagement.
In this article, we’ll define what barcodes are in the context of 2025, explore how they function today, and discuss their growing importance in modern business and technology.
What is a Barcode in 2025?
A barcode is a machine-readable representation of data that encodes information into a visual pattern. Traditionally, barcodes used vertical lines (1D codes), but in 2025, 2D barcodes like QR codes, Data Matrix, and PDF417 have become standard in many industries.
Barcodes now serve as digital identifiers that connect physical objects to cloud-based data systems in real time. They're used for product tracking, authentication, traceability, marketing, and more.
How Barcodes Work in 2025
The basic principle remains the same: a barcode scanner reads the visual pattern and decodes it into usable data. However, in modern times, several advancements have transformed how barcodes are created, scanned, and used.
1. Modern Barcode Scanners
Devices now include smartphones, AI-enabled scanners, and IoT sensors.
Cameras and software can instantly scan multiple barcodes in one go.
Cloud integration means data is processed in real-time and accessible from anywhere.
2. Mobile and Cloud Integration
Scanned data is instantly uploaded to cloud-based inventory or ERP systems.
Businesses use barcode apps for real-time tracking, analytics, and automation.
APIs allow barcodes to interact with customer apps, POS systems, and logistics software.
3. 2D Barcode Expansion
QR codes and Data Matrix codes can store more data in less space.
They can encode URLs, product details, tracking IDs, and even dynamic content.
Often used in digital menus, vaccine passports, shipping labels, and smart packaging.
4. AI & Machine Learning Integration
AI enhances barcode recognition under poor lighting, angle distortion, or damaged labels.
Machine learning algorithms predict inventory needs based on barcode scan history.
5. Augmented Reality (AR) & Barcodes
Some systems allow workers to scan barcodes through AR glasses, showing item details instantly.
This improves picking speed and accuracy in large warehouses.
Types of Barcodes in 2025
TypeDescriptionCommon Use CasesUPC (1D)Basic linear barcodeRetail products, groceriesCode 128 (1D)High-density, alphanumericShipping, logisticsQR Code (2D)Square code, stores more dataMarketing, payments, authenticationData MatrixCompact 2D codePharmaceuticals, electronicsPDF417Stacked linear barcodeIDs, boarding passes, documents
Benefits of Modern Barcodes
✅ Real-Time Data Access
Scanned data is synced across platforms instantly, improving visibility and decision-making.
✅ High-Speed Scanning
Advanced scanners can read dozens of barcodes simultaneously, even from a distance or on moving packages.
✅ Enhanced Traceability
Used extensively in food, pharma, and logistics industries to track items from origin to delivery.
✅ Improved Customer Experience
QR codes connect products to rich media, reviews, promotions, or authentication tools.
✅ Cost-Effective & Scalable
Barcodes are low-cost to print or display and scale easily across large inventories or product lines.
Barcode Use Cases in 2025
● Retail & eCommerce
Dynamic pricing via barcode-linked software
Self-checkout systems using smartphone barcode scans
● Healthcare
Barcode wristbands for patients
Tracking medication, lab samples, and medical equipment
● Manufacturing
Parts traceability throughout production lines
Quality control through scan-and-check systems
● Logistics & Supply Chain
Real-time shipment tracking
Warehouse automation using barcode-based inventory robots
● Education & Events
Digital student IDs with QR codes
Entry passes and attendance via barcode scans
The Evolution of Barcodes: 2025 and Beyond
Barcodes are now a bridge between physical objects and digital ecosystems. As IoT, blockchain, and AI continue to grow, barcodes will serve as gateways for secure, real-time data exchange. In fact, smart barcodes embedded in sustainable packaging or NFC-enabled tags are already being tested to go beyond basic scanning.
Some emerging innovations include:
Dynamic barcodes that change after each scan to prevent fraud.
Biometric-linked barcodes for secure identity verification.
Voice-activated barcode scanning integrated with smart assistants.
Conclusion
Barcodes in 2025 are smarter, faster, and more connected than ever before. From retail shelves to hospital rooms, they continue to drive efficiency, accuracy, and innovation across industries. As businesses adapt to an increasingly digital and automated world, barcodes remain a simple yet powerful tool to bridge the physical and virtual realms.
Looking to upgrade your inventory or product tracking with advanced barcode solutions? Contact us today to get started with modern barcode systems built for 2025 and beyond.
#rfid solutions#asset management#real time tracking#barcode#aidc technologies india#aidc#electronic devices#technology#qr code#barcode printers
0 notes
Text
Enzymes in Pharma: Unlocking New Frontiers in Drug Discovery and Treatment

Pharmaceutical enzymes are revolutionizing the drug development and therapeutic innovation landscape. These strong biological catalysts catalyze key reactions with accuracy, providing novel solutions for the pharmaceutical industry.
Why Pharmaceutical Enzymes Matter
Pharmaceutical enzymes are a part of the resurgence in the use of enzymes. They play a crucial role in speeding up drug synthesis, enhancing yield, and lowering costs in production. Their use in biocatalysis means greener and more sustainable production techniques. Right from producing active pharmaceutical ingredients (APIs) to developing enzyme-based therapeutics, enzymes facilitate lab-to-market progress.
Enzyme-Based Therapeutics: A New Era
Enzyme technology is one of the latest advances in contemporary medicine. Therapeutic enzymes are successfully applied to the treatment of genetic diseases, metabolic disorders, and even cancers. For instance, enzyme replacement therapies provide revolutionary treatments for rare disease patients suffering from lysosomal storage disorders.
Innovation with Enzyme Technology
Pharmaceutical firms are using enzyme technology to design targeted drug delivery systems, improve drug solubility, and minimize side effects. The specificity and precision of enzymes are well suited for creating safer and more effective treatments.
Future Directions
With advancements in research, pharmaceutical enzymes will increasingly open up new avenues in personalized care and regenerative medicines. Enzyme-based solutions are opening up new avenues towards greater accessibility and ease in healthcare.
Discover how enzyme technology can drive your pharma innovations. Lead the way in the age of enzyme-based medicines.
Read also:https://ultrezenzymes.com/pharmaceutical-enzymes-drug-development/
0 notes
Text
Caffeine in the USA | Chemical Properties, Market Size, and Regulatory Landscape
Caffeine is a familiar friend whose usefulness can hardly be overestimated, it is taken in the morning and during working hours to prevent the sense of fatigue. This psychoactive stimulant used in drinks as coffee, tea, energy drinks and in various dietary supplements has attracted consumers worldwide. It is a complex compound, which has a multi-faceted role in our daily lives, that is why caffeine an indispensable part of our diets and a major component in the global economy. In this blog, we look at the basic details about caffeine including its chemical properties, the growth in global market size over the years and the current rules governing it in the United States.
#low cost medicine#pharmacy distributor#API meaning pharma#top pharma companies in USA#contract manufacturing pharma
0 notes
Text
Fosfomycin Calcium Market Global Opportunity Analysis & Industry Forecast, 2024–2030.
Fosfomycin Calcium Market Overview:

Request Sample:
Fosfomycin calcium is witnessing increased demand due to its application as an adjuvant in chemotherapy treatments especially with the growing incidence of cancer. According to the World Health Organisation, globally over 35 million new cancer cases are predicted in 2050. The role of fosfomycin in combating bacterial infections during chemotherapy makes it a valuable supportive therapy enhancing patient outcomes by addressing infection risks that arise from compromised immune systems. Additionally, fosfomycin calcium aligns with global antibiotic stewardship programs aimed at curbing antibiotic resistance. These programs advocate for the selective use of antibiotics with broad-spectrum efficacy, such as fosfomycin, which has shown effectiveness against multidrug-resistant bacteria.
Impact of Covid and Russia Ukraine War:
The COVID-19 pandemic handicapped the global market extensively resulting in several challenges most notably, supply chain disruptions, but the pandemic caused an upsurge in the sales of the pharma industry. With increasing number of lifestyle diseases, there was a substantial demand for drugs during the pandemic, particularly diabetes medication.
The ongoing Ukraine-Russia conflict’s impact on Fosfomycin Calcium is indirect. Disruptions in supply chains due to the conflict led to delays in shipments of these drugs. Additionally, geopolitical tensions affect the manufacturing and distribution of the product, causing fluctuations in market availability, pricing, and international trade dynamics within the industry.
Key Takeaways:
APAC is the largest Market
Geographically, APAC held the largest share with 42% of the overall market in 2023 and it is poised to dominate the market over the period 2024–2030. The APAC region faces a higher incidence of bacterial infections, which often require antibiotics like Fosfomycin Calcium. Some APAC countries are major manufacturers and exporters of pharmaceuticals. This contributes to the availability and accessibility of Fosfomycin Calcium in the region. Most of the leading producers of Fosfomycin are located in the APAC region only. India is the source of 60,000 generic brands across 60 therapeutic categories and manufactures more than 500 different Active Pharmaceutical Ingredients (APIs), as per Invest India. The export of generic drugs is one of India’s core strengths. According to India Brand Equity Foundation, the total annual turnover of the Indian Pharmaceutical Industry reached $49.8 billion in FY23 and $41.7 billion in 2021–22.
Inquiry Before Buying:
By Form, Tablet is the Largest Segment
Tablets are a convenient and portable dosage form. They’re easy to store, transport, and take with or without water, making them patient-friendly. Tablets can be precisely manufactured to contain a specific dose of Fosfomycin Calcium, ensuring consistent medication delivery. Compared to other formats like injections or liquids, tablets can be a more cost-effective option to manufacture and distribute. Fosfomycin Calcium for UTIs is often prescribed as a single-dose treatment. Tablets are well-suited for this purpose, as they offer a simple and complete dose in one unit. Tablets are the most widely prescribed dosage form as they are cost-effective, stable, and easy-to-administer. According to the Japan Pharmaceutical Manufacturers Association (JPMA), tablets accounted for 46.5%, followed by injections (13.9%). These two categories made up 60.4% of the total amount of production of pharmaceuticals in 2020 by drug form.
Urinary Tract Infection is the Largest Segment
Urinary tract infections (UTIs) are extremely common, especially among women. This high prevalence translates to a significant market for UTI treatments. Fosfomycin is a broad-spectrum antibiotic, meaning it targets a wide range of bacteria that can cause UTIs. This makes it a valuable option for treating UTIs caused by susceptible strains, including E. coli, a common culprit. Unlike many other antibiotics that require multiple doses over several days, Fosfomycin Calcium for UTIs often comes as a single-dose treatment.
Schedule A Call:
This improves patient compliance and reduces the risk of missed doses or incomplete treatment. According to a report in the research square, In 2019, more than 404.6 million (95% UI 359.4–446.5) individuals had UTIs globally and nearly 236 786 people (198 433 − 259 034) died of UTIs, contributing to 5.2 million (4.5–5.7) DALYs. The age-standardised incidence rate increased from 4 715.0 (4 174.2–5 220.6) per 100 000 population in 1990 to 5 229.3 (4 645.3–5 771.2) per 100 000 population in 2019.
Growing Pharmaceutical Industry Drives the Market
The pharmaceutical industry is flourishing due to the outbreaks of diseases. With the global burden of diseases growing, there is a need for pharmaceuticals to treat diseases. According to Invest India, the pharmaceutical industry in India is expected to reach $65 billion by 2024 and to $130 billion by 2030. India is a major pharmaceutical exporter, supplying over 200 countries, including meeting 50% of Africa’s generic drug needs, 40% of the U.S. demand, and 25% of the UK’s demand. Additionally, the growing antibiotic resistant has led to the search for potent compounds and fosfomycin’s ability to treat infections caused by resistant bacteria is invaluable.
Regulatory Compliance to Hamper the Growth
Buy Now :
Regulations govern the information that can be included on the packaging and prescribing information for Fosfomycin Calcium medications. A prime example is the European Medicines Agency’s (EMA) 2020 recommendations limiting the use of fosfomycin-containing medications. This restricted the use of intravenous Fosfomycin Calcium to serious infections only. EMA has recommended that fosfomycin medicines given by infusion (drip) into a vein should only be used to treat serious infections when other antibiotic treatments are not suitable. Fosfomycin medicines given by mouth can continue to be used to treat uncomplicated bladder infections in women and adolescent girls. Such regulations can significantly impact market growth in affected regions.
For more Lifesciences and Healthcare Market reports, please click here
#FosfomycinCalcium#PharmaceuticalMarket#AntibioticsIndustry#HealthcareTrends#MarketAnalysis#PharmaBusiness#GlobalHealthcare#DrugDevelopment
0 notes
Text
DISCOVERY RESEARCH ‘FINDS’ A MOLECULE
In most industries, supply chains are created as part of a new product development program. The developers work together, consulting product end-users, producers, and distributors to ensure the physical supply chain they put together can deliver what is required by consumers.
For reasons we shall go into later, pharmaceutical new product development does not follow the customary approach of other industries. Rather than beginning with a consumer and working upstream to the beginning, the process begins with a patented compound and moves forwards.
This results in supply chains ‘evolving’ with the passage of time, rather being designed and planned with the consumer in mind.
The ‘R’ of R&D (Discovery Research) discovers (or finds) molecules using advanced technologies such as molecular modelling. Once it is confirmed a patent is in place, promising compounds are handed to ‘D’ (Development).
PRODUCT DEVELOPMENT HAS TO FILE IT WITH THE REGULATORS
Development is a completely new team of specialists responsible for building a supply chain for pre-clinical testing initially. That means proving the test compound that will be produced by the supply chain is safe to study in humans. For small molecule products (made by industrial chemistry), around 5 – 10 kilograms of compound will be produced.
Figure 5 shows a typical small molecule product supply chain for the production of the active pharmaceutical ingredient (API).
0 notes
Text
Kekule Pharma Limited: Leading API and Intermediates Manufacturer and Supplier in India
API and Intermediates – Leading Manufacturer and Supplier in India
India has become a global powerhouse in pharmaceutical manufacturing, contributing significantly to the supply of Active Pharmaceutical Ingredients (APIs) and Intermediates. Among the leading names in the industry, Kekule Pharma Limited stands out as a trusted partner for its commitment to quality, innovation, and customer satisfaction.
API Manufacturers in India
As a top player in the industry, Kekule Pharma Limited specializes in producing high-quality APIs for various therapeutic segments, including oncology, cardiovascular, and anti-infective drugs. With decades of experience, Kekule leverages advanced technology, rigorous quality control, and a dedicated team of experts to deliver reliable and cost-effective solutions.
Indian API manufacturers, including Kekule, are recognized for their ability to meet stringent global standards, ensuring compliance with regulations such as USFDA, WHO-GMP, and EU guidelines. This commitment to quality has made India a preferred destination for pharmaceutical companies worldwide.
Intermediates Manufacturers in India
Kekule Pharma Limited is also a leading name among Intermediates manufacturers in India. Intermediates are the foundation for synthesizing complex APIs, and Kekule excels in multistage synthesis to deliver high-purity intermediates. The company’s infrastructure and expertise ensure it meets the growing demands of the pharmaceutical and chemical industries, both locally and globally.
Why Kekule Pharma Leads in APIs and Intermediates
Expertise in Multistage Synthesis: Kekule’s innovative approach to chemical engineering ensures the efficient production of complex APIs and Intermediates.
State-of-the-Art Infrastructure: The company’s facilities are equipped with world-class technology to deliver scalable solutions.
Global Standards Compliance: Kekule’s commitment to quality is reflected in its adherence to international regulatory requirements.
Customer-Centric Approach: With a focus on long-term partnerships, Kekule delivers cost-effective, high-quality solutions tailored to customer needs.
API Suppliers in India
As a reliable API supplier in India, Kekule Pharma Limited ensures timely delivery and uncompromising quality. The company’s robust supply chain and customer-first philosophy have positioned it as a trusted partner for pharmaceutical companies worldwide.
Intermediates Suppliers in India
Kekule Pharma Limited also excels as a supplier of intermediates, providing customized solutions for diverse applications. Its focus on innovation and process optimization enables clients to achieve their goals efficiently and cost-effectively.
Partner with Kekule Pharma Limited
Choosing Kekule Pharma Limited as your API and Intermediates partner means aligning with a company that prioritizes quality, innovation, and integrity. Backed by nearly four decades of experience, Kekule is equipped to handle complex chemical challenges while meeting tight deadlines and stringent quality requirements.
With a customer-centric approach, Kekule Pharma Limited delivers:
High-Quality APIs and Intermediates for a wide range of therapeutic segments.
Tailored Solutions to meet unique client requirements.
On-Time Delivery and reliable project management.
As one of the leading API manufacturers in India and Intermediates suppliers in India, Kekule Pharma Limited is committed to advancing pharmaceutical innovation while maintaining affordability and excellence.
0 notes
Text
Navigating the Future of Pharma: How BizMagnets Outperforms Tata 1mg ?

Ready to boost your Business with BizMagnets ?
Contact us for a demo, and let’s start your journey towards enhanced customer engagement, streamlined processes, and increased revenue.
Introduction:
In a time when digital platforms are changing how people access healthcare, pharmacies need to use new and innovative solutions to stay ahead in the market. 1mg has been a leader in providing digital pharmacy services, but a new platform called BizMagnets WhatsApp Business Suite is offering an interesting alternative, especially when it comes to providing personalized and efficient customer service
The Advantage of the WhatsApp Business API
WhatsApp has a huge number of users all around the world, which makes it a great way for pharmacies to communicate with and engage their customers in a more personal way. The BizMagnets suite, which uses the WhatsApp Business API, allows pharmacies to not only reach their customers but also really connect with them. This can provide services that go beyond what other online pharmacy platforms like 1mg currently offer.
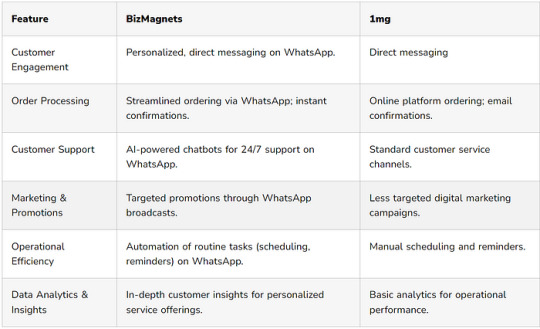
How WhatsApp Business API Can Transform Your Pharmacy Business ?
1. Personalized Customer Journeys : The BizMagnets platform uses AI to provide each customer with a customized experience, which can help build customer loyalty and encourage repeat business, unlike the more generic interactions on platforms like 1mg.
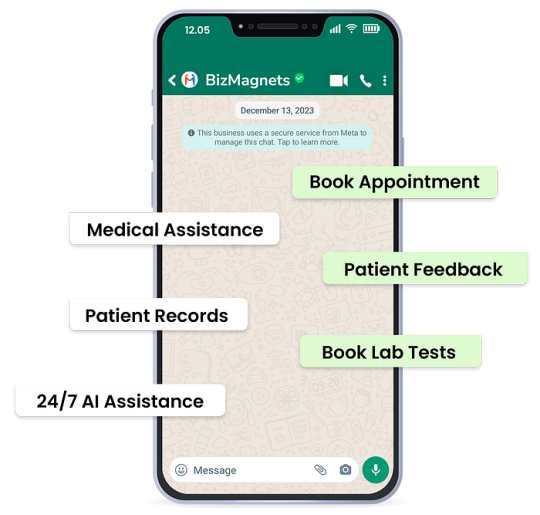
2. Efficient Operations : Automating tasks like appointment booking, test scheduling, and order confirmations through WhatsApp can save time and reduce errors, making pharmacy operations more efficient.
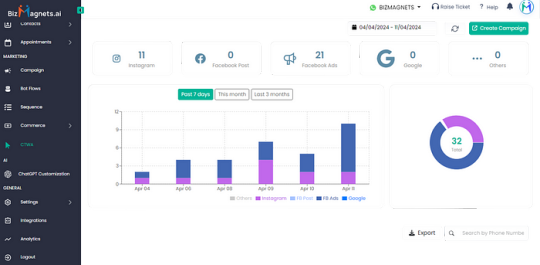
3. Data-Driven Decisions : The advanced analytics capabilities of the BizMagnets platform allow pharmacies to gain valuable insights into customer behavior, which they can then use to make more informed business decisions and tailor their services more precisely
Provoking Thoughts
How could personalized WhatsApp messages transform your pharmacy’s customer service experience?
Imagine the efficiency gains from automating routine operations through WhatsApp. How would that change the day-to-day of your pharmacy?
With the insights provided by BizMagnets, what new services or products could you offer to meet the unique needs of your customers?
For a more in-depth analysis on how the WhatsApp Business API can empower pharmacies to outshine competitors like Tata 1mg through specific features like WhatsApp broadcast, drip campaigns, and Click to WhatsApp ads, let’s expand on each of these components, highlighting their benefits and potential impact on business growth
WhatsApp Broadcast: The Power of Personalized Messaging at Scale
WhatsApp Broadcast allows businesses to send messages to multiple customers at once, provided the customers have saved the business’s phone number in their contacts and have agreed to receive messages. This feature is pivotal for pharmacies in announcing new health products, vaccine availability, or seasonal health tips directly through WhatsApp, ensuring high visibility and engagement. Unlike 1mg’s approach, which may rely more on app notifications or emails, WhatsApp broadcasts feel more personal and are likely to be read by customers
Drip Campaigns: Nurturing Customer Relationships Over Time
Drip campaigns are automated sets of messages that are sent out based on specific timelines or user actions. For pharmacies leveraging BizMagnets, this means being able to automatically send a welcome series to new subscribers, educational content on managing chronic conditions, or reminders for prescription refills. This strategic communication keeps the pharmacy top of mind for customers and can encourage repeat purchases. Drip campaigns through WhatsApp can be more effective than traditional methods used by companies like Tata 1mg, due to the personal and immediate nature of messagingw
Click to WhatsApp Ads: Driving Conversations and Conversions
Click to WhatsApp ads are a powerful tool that integrates with Facebook and Instagram advertising platforms. When users click on an ad, they are directly taken to a WhatsApp conversation with the business. For pharmacies, this means being able to advertise specific products or health services and instantly engage with interested customers, providing personalized advice or facilitating orders directly through WhatsApp. This immediate engagement model can significantly outperform the more static online purchasing experience offered by platforms like 1mg, leading to higher conversion rates and customer satisfaction
The Competitive Edge
Implementing these features through the WhatsApp Business API offers a dynamic and interactive customer experience that stands in contrast to the more traditional, website-centric approach of competitors like 1mg
Here’s how:
Enhanced Personalization: WhatsApp allows for direct, one-on-one communication, making each customer feel valued and understood.
Higher Engagement Rates: Messages on WhatsApp have a higher open and read rate compared to emails and app notifications.
Path to Purchase: By reducing the steps needed to inquire or purchase, customers are more likely to complete transactions.
Empowering Businesses to Thrive in the Digital Age with BizMagnets
In today’s fast-paced, technology-driven business world, adapting and innovating is crucial. By partnering with BizMagnets and leveraging the power of the WhatsApp Business API, companies can gain a significant competitive edge. The BizMagnets.ai platform offers an AI-driven WhatsApp Business Suite that empowers businesses to provide personalized, efficient, and seamless customer communication.
This innovative solution allows companies to meet their customers where they are and deliver a superior customer experience It can gather essential information from leads based on predefined criteria, ensuring that sales teams focus their efforts on high-potential prospects.
Visit: https://bizmagnets.ai/navigating-the-future-of-pharma-how-bizmagnets-outperforms-1mg-with-bizmagnets/
Email: [email protected]
Contact Number: 7845079333
#tata 1mg#medical chatbot#pharmacy#health#technology#whatsapp api#chatbot#whatsapp business#whatsapp api provider#whatsapp flows#business#chatgpt#healthcare chatbots market#whatsapp business api#saas#b2b saas#saas technology#saas software#artificial intelligence#tata#1mg
0 notes